UK MHRA grants authorisation for Moderna’s Covid-19 booster vaccine
Pharmaceutical Technology
AUGUST 15, 2022
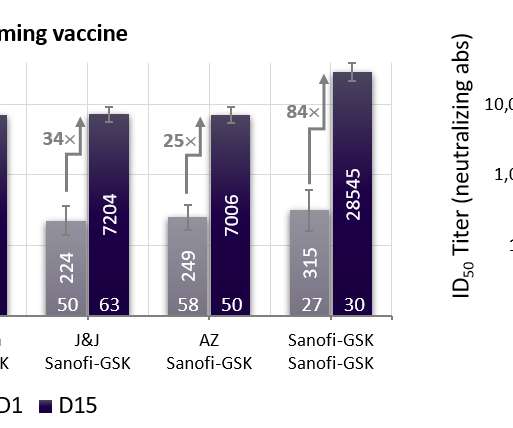
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted conditional authorisation for Moderna ’s Covid-19 booster vaccine, mRNA-1273.214 (Spikevax Bivalent Original/Omicron), for use in adults aged 18 years and above. It comprises mRNA-1273 (Spikevax) and a vaccine candidate that acts on the SARS-CoV-2 virus’ BA.1






































Let's personalize your content