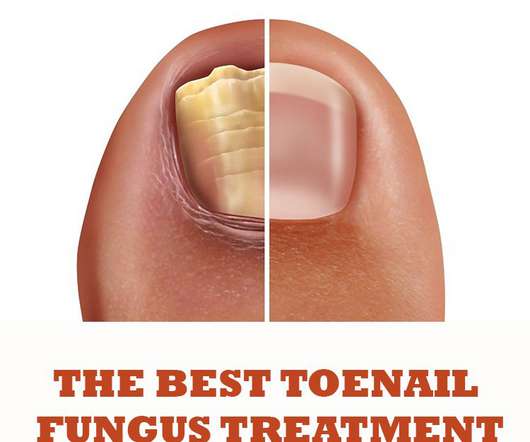
Best Toenail Fungus Treatment Products
Druggist
OCTOBER 21, 2020
It is recommended that a particular group of people; for example, diabetic patients seek advice from a podiatrist or diabetic nurse as any infections in diabetes may lead to complications. Topical products, which are applied directly to the infected area, are the main products used as toenail fungus treatment.














Let's personalize your content