Prefilled syringes and vaccines: Optimising parenteral packaging for the next pandemic
Pharmaceutical Technology
NOVEMBER 15, 2022
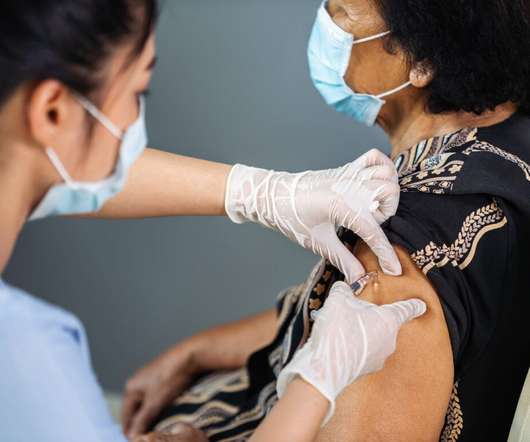
When it comes to vaccines and other high-value biologics, it is essential that effective formulations go hand in hand with safe delivery. By having the drug already dosed inside the chamber and no need to draw it from a vial into a syringe, the process is streamlined and the risk of a potential dosing error is eliminated.












Let's personalize your content