BioSig abandons COVID-19 drug trial on safety concerns
pharmaphorum
OCTOBER 27, 2020
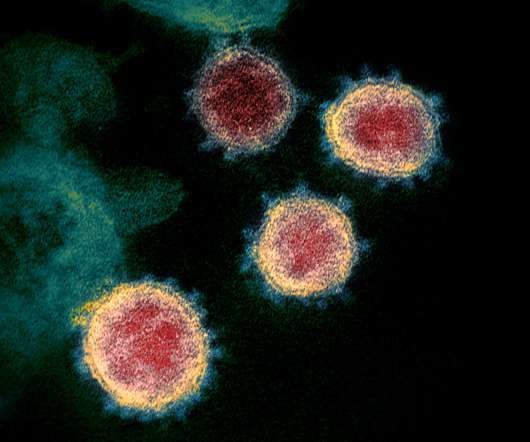
US biotech BioSig Technologies has abandoned a phase 2 trial of its antiviral drug merimepodib with Gilead’s Veklury in severe COVID-19 patients, after concluding the safety of the combination was in doubt. Merimepodib is the subsidiary’s primary asset, and the biotech now says it is to offers to acquire or license the drug.








Let's personalize your content