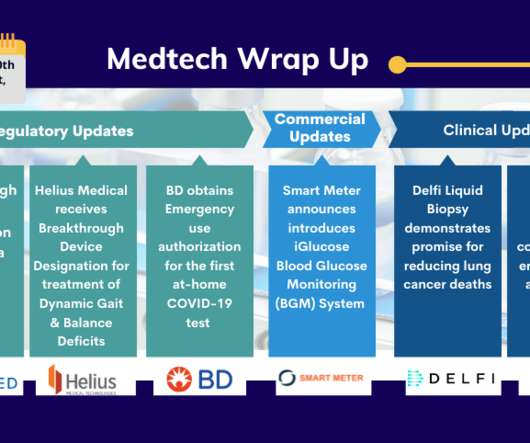
Abiomed’s Impella ECP; Helius’s PoNS Device; Smart Meter’s iGlucose BGM; XACT’s CT-Guided Percutaneous Procedures; BD’s Veritor At-Home COVID-19 Test; Delfi’s Liquid Biopsy
Delveinsight
SEPTEMBER 2, 2021
On August 18, 2021, Abiomed received breakthrough device designation from the United States Food and Drug Administration (FDA) for its Impella ECP expandable percutaneous heart pump , the world’s smallest heart pump and the first to be compatible with small-bore access and closure techniques.









Let's personalize your content