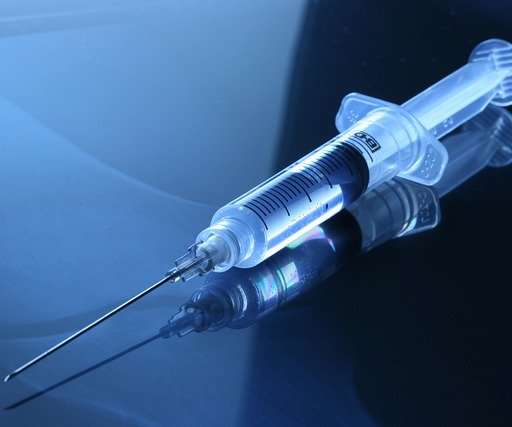
Xolair (omalizumab) Prefilled Syringe for Self-Injection Across All Indications
The Pharma Data
APRIL 14, 2021
Food and Drug Administration (FDA) has approved the company’s supplemental Biologics License Application for Xolair® (omalizumab) prefilled syringe for self-injection across all approved U.S. with Xolair since its initial approval in 2003. today announced that the U.S. indications. In the U.S., Pulmozyme ® ?(dornase?alfa)

















Let's personalize your content