Funding Rehab: Knowing Your Options
Pharma Mirror
MAY 1, 2024
Addiction is affecting a large portion of people’s lives at the minute, but one of the things people often struggle with is knowing how to afford rehab. There’s a common misconception that it costs thousands upon thousands of pounds and is entirely unattainable for people in some circles of society. However, that’s precisely what it is, a misconception, and many options can help people, no matter how wealthy or poor they are.













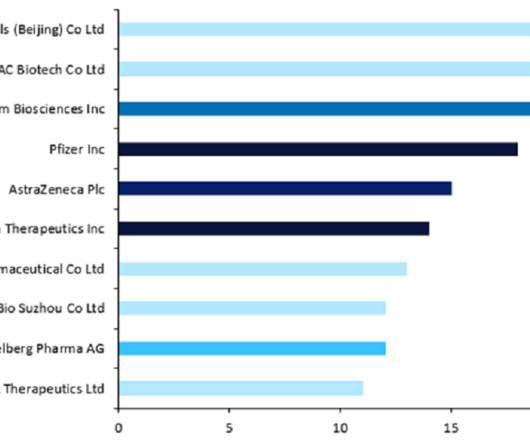





























Let's personalize your content