FDA rejects Provention’s type 1 diabetes drug teplizumab
pharmaphorum
JULY 7, 2021
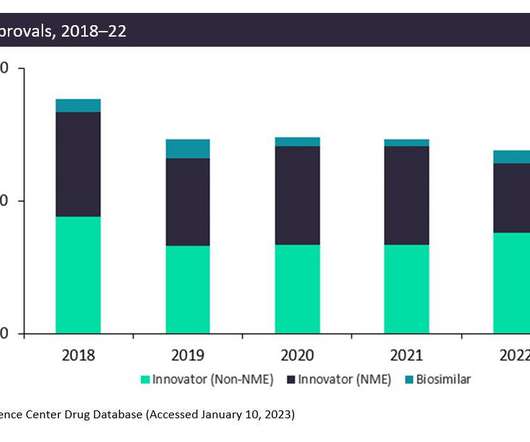
The complete response letter (CRL) for teplizumab as a treatment to delay clinical type 1 diabetes in at-risk individuals reiterates the FDA’s concerns around the pharmacokinetics (PK) data for the antibody.









Let's personalize your content