Lumen Bioscience’s LMN-201 secures FDA fast track status to treat CDI
Pharmaceutical Technology
MAY 18, 2023
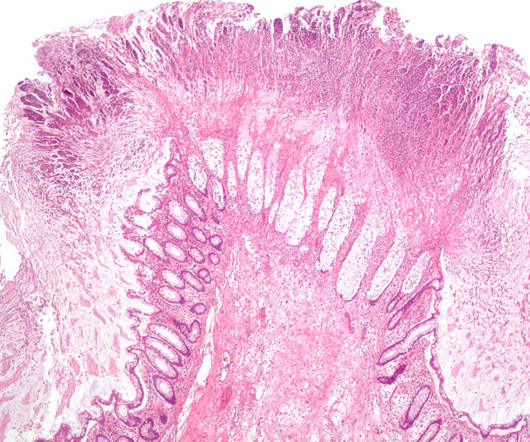
Biotechnology company Lumen Bioscience has secured fast track designation from the US Food and Drug Administration (FDA) for LMN-201 for the treatment and prevention of C difficile infection (CDI). It is compatible with standard-of-care antibiotics. Pivotal trials on LMN-201 are expected to commence in 2023.











Let's personalize your content