Bluebird preps for first commercial use of sickle cell gene therapy
Bio Pharma Dive
MAY 6, 2024
A 12-year-old boy in the Washington, D.C., area is set become the first patient treated with Lyfgenia since its U.S. approval last December.

Bio Pharma Dive
MAY 6, 2024
A 12-year-old boy in the Washington, D.C., area is set become the first patient treated with Lyfgenia since its U.S. approval last December.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 6, 2024
Aimed at driving innovation and progress in the field of microbiology, the Indian Pharmacopoeia Commission (IPC) and the CSIR-Institute of Microbial Technology (IMTech), Chandigarh, have joined forces towards advancing microbiological research and development for the betterment of public health.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
MAY 6, 2024
With many chemotherapy and ADHD drugs stuck in stubbornly short supply, several agencies are looking for new solutions.
Pharmaceutical Technology
MAY 6, 2024
The UK MHRA approved two formulations of ViiV Healthcare’s cabotegravir, offering a new prevention option for HIV-1 infection.

Bio Pharma Dive
MAY 6, 2024
One analyst thinks the collaboration “bodes well” for Gossamer, which has had trouble convincing investors its drug seralutinib can compete on the market.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 6, 2024
People have a pretty intuitive sense of what is healthy – standing is better than sitting, exercise is great for overall health and getting good sleep is imperative.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
MAY 6, 2024
Lantern Pharma has entered into a partnership with Oregon for optimising the development of XCE853, through an AI-driven approach.

Bio Pharma Dive
MAY 6, 2024
The ability to modify drug formulations in real time and dosage strengths is crucial, while adhering to all regulatory standards and maintaining cGMP standards.

Pharmaceutical Technology
MAY 6, 2024
Sentynl Therapeutics has announced the acquisition of Zokinvy programme from Eiger BioPharmaceuticals for the treatment of progeria.

Fierce Pharma
MAY 6, 2024
As BioNTech continues to endure a sharp decline in COVID-19 vaccine sales, the German mRNA specialist is looking ahead to the next leg of its commercial journey. | With plans to have at least 10 potentially registrational trials underway by the end of 2024, BioNTech is plotting the first wave of its market debut in oncology from 2026 onward.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
MAY 6, 2024
Cipla and Glenmark Pharmaceuticals are recalling specific products from the US market due to distinct manufacturing issues.

Bio Pharma Dive
MAY 6, 2024
Optimized clinical trials will help sponsors align with new FDORA diversity requirements.

Fierce Pharma
MAY 6, 2024
Amneal Pharmaceuticals has agreed to pay $272.5 million to settle approximately 900 lawsuits brought by state, local and Native American jurisdictions claiming that the New Jersey generics producer | Amneal Pharmaceuticals has agreed to pay $272.5 million to settle approximately 900 lawsuits brought by state, local and Native American jurisdictions claiming that the New Jersey generics producer fueled the opioid crisis by failing to act on suspicious opioid orders.

Pharmaceutical Commerce
MAY 6, 2024
Radio frequency identification (RFID) not only helps hospitals boost their medication safety standards, as it also allows clinicians to continue to provide quality patient care.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

BioSpace
MAY 6, 2024
Jeffrey Chamberlain, president of the American Society of Gene & Cell Therapy, spoke with BioSpace about what we can expect to learn about in Baltimore this week.

FDA Law Blog
MAY 6, 2024
By Lisa M. Baumhardt, Senior Medical Device Regulation Expert & Adrienne R. Lenz, Principal Medical Device Regulation Expert — FDA recently issued a draft guidance which would update the agency’s Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions guidance. The draft guidance provides recommendations on what is required to meet cybersecurity obligations under section 524B of the Food, Drug and Cosmetic Act (FD&C).

BioSpace
MAY 6, 2024
BioSpace will be in attendance at the American Society of Gene & Cell Therapy’s 27th annual meeting, along with thousands of others. Stay with us for updates throughout the week.

ACRP blog
MAY 6, 2024
On Day Two of ACRP 2024 in Anaheim, Calif., attendees were invited to take advantage of “the power of pause” to calm down their overstressed and nonstop brains, while on Day Three, they were encouraged to think of each day as Day One for making the kinds of incremental changes that will lead to clinical research being recognized widely as profession and clinical trials as a clinical care option.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
MAY 6, 2024
“Our next question will come from Andrew Baum with Citi.” | Citi analyst Andrew Baum, after covering Pfizer for more than a decade, will join the Big Pharma company as chief strategy and innovation officer. In the role, he'll oversee the drugmaker's portfolio management and business development activities.

Bio Pharma Dive
MAY 6, 2024
Can free bioinformatics tools get the job done?

BioSpace
MAY 6, 2024
FDA Commissioner Robert Califf has said that advisory committee meetings should focus more on discussion and be “less about the outcome,” but other agency officials recently advocated for retaining the vote on whether to approve a product under review.

pharmaphorum
MAY 6, 2024
The Novo Nordisk Foundation, the Bill & Melinda Gates Foundation, and Wellcome have joined forces to tackle some of the most pressing threats to human health and wellbeing. The new initiative gets underway with $300 million in funding over three years earmarked for handling infectious diseases and antimicrobial resistance (AMR), addressing climate change, and raising our understanding of how nutrition can impact immunity, disease, and the development of children.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

BioSpace
MAY 6, 2024
Adeno-associated viruses have long been go-to vectors for gene therapies. How AAVs are improving will be among the cell and gene therapy topics to be covered in Baltimore this week.
Fierce Pharma
MAY 6, 2024
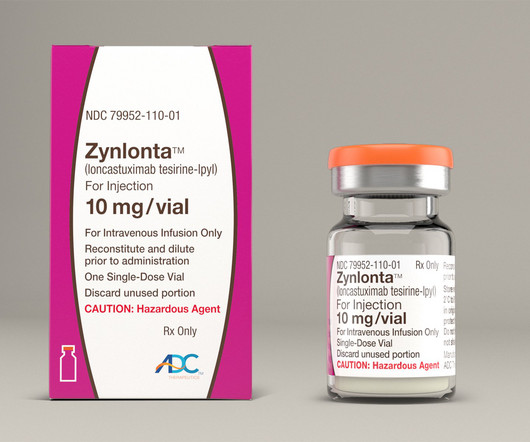
In a flurry of updates Monday, ADC Therapeutics revealed that it's selling $105 million in new shares while also touting results for antibody-drug conjugate Zynlonta from a small investigator-initi | ADC Therapeutics provided several company updates Monday, including that it's raising $105 million through a sale of common shares and pre-funded warrants.

BioSpace
MAY 6, 2024
The Italian pharma will gain access to Gossamer Bio’s candidate seralutinib, which reached its primary endpoint in a Phase II pulmonary arterial hypertension trial in 2022 and started a Phase III study last year.

pharmaphorum
MAY 6, 2024
Boehringer drug shows promise in diabetic macular ischaemia (DMI), a common and irreversible complication of the eye in people with diabetes with no approved therapies

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
MAY 6, 2024
Q&A: Development Scientist at AGC Biologics Sara Morlacchi analyzes the growth of the cell therapy industry and barriers for cost and accessibility.

pharmaphorum
MAY 6, 2024
Pfizer has named Citi analyst Andrew Baum as its chief strategy and innovation officer, taking point on portfolio management and capital deployment

BioSpace
MAY 6, 2024
With three FDA approvals in the past 10 months, there is a lot of momentum in the Duchenne muscular dystrophy space. Here are five companies looking to keep it going.

pharmaphorum
MAY 6, 2024
Shah Capital has stepped up its campaign for change at vaccine producer Novavax, lobbying against the re-election of three directors and executive pay packages in a letter to fellow stockholders. The hedge fund says it wants to send a “strong and clear message” to the board at Novavax, reiterating the claim – first made a few weeks ago – that the company is “being hindered by an overly conservative board and management that clings to failed strategies.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content