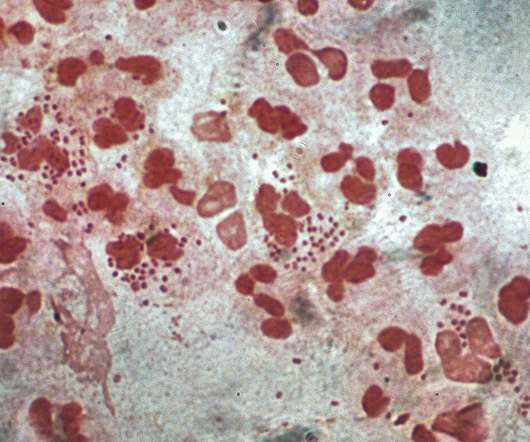
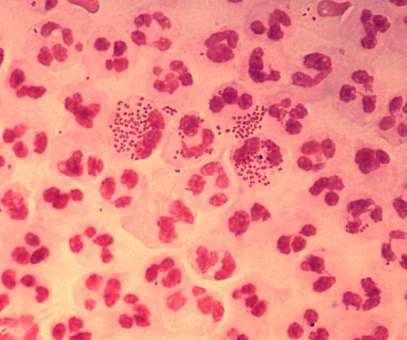
Intravacc gets NIAID contract for intranasal gonorrhoea vaccine development
Pharmaceutical Technology
OCTOBER 6, 2022
Intended for intranasal administration, NGoXIM was demonstrated to be effective in animal models and elicited a durable and cross-protective immune response in proof-of-concept (PoC) studies. . Therapyx will develop and manufacture the IL-12-containing microspheres in the vaccine called GneX12.









Let's personalize your content