Covid-19 Omicron boosters will reinvigorate injectables manufacturing
Pharmaceutical Technology
JULY 29, 2022
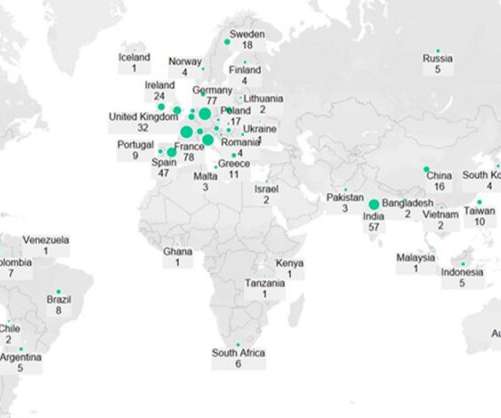
Pfizer (New York, New York) and Moderna’s (Cambridge, Massachusetts) Omicron-specific Covid-19 vaccines are in late-stage development, have demonstrated good efficacy against the variant and will likely be available to the public later this year to provide an additional booster and increase demand for injectable manufacturing.








































Let's personalize your content