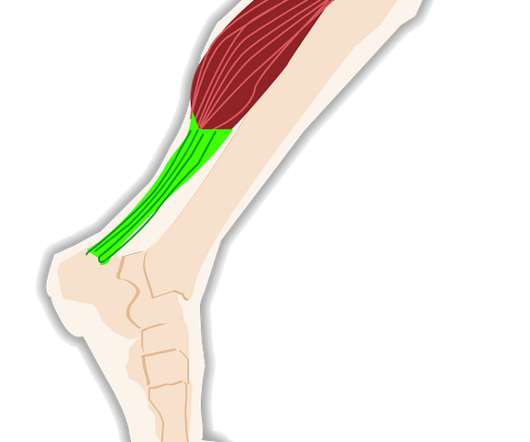
FDA Approves Expanded BOTOX® (onabotulinumtoxinA) Label to Include Eight New Muscles to Treat Adults with Upper Limb Spasticity
The Pharma Data
JULY 29, 2021
are living with spasticity across a variety of neurologic conditions — BOTOX® has demonstrated efficacy and has an established safety profile with over 10 years of clinical use in adult upper limb spasticity. Senior Vice President, Chief Scientific Officer, BOTOX ® & Neurotoxins, AbbVie. million adults in the U.S.





















Let's personalize your content