Unpacking the Financial Implications of EDC System Implementation: Initial Costs, Long-term Savings, and ROI
Cloudbyz
JUNE 2, 2023
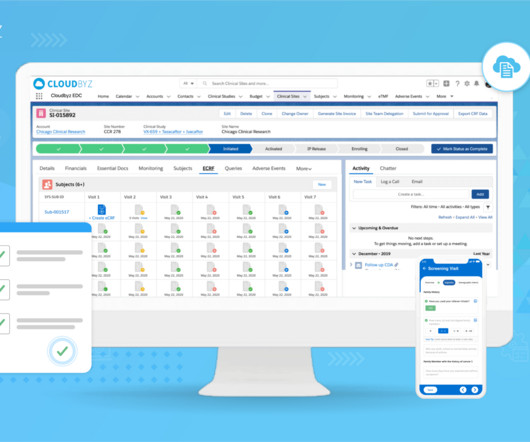
Electronic Data Capture (EDC) has revolutionized clinical research and pharmaceutical drug development. However, adopting this system requires a significant financial commitment. Initial Costs The initial costs of implementing an EDC system can be substantial, encompassing both direct and indirect expenses.










Let's personalize your content