Pharmaceutical compliance management software for the pharmaceutical industry
Pharmaceutical Technology
APRIL 19, 2024
Pharmaceutical Compliance Management Software Is Designed to Enhance Patient Safety. Download the Free Buyer’s Guide Here.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 Compliance Related Topics
Compliance Related Topics 
Pharmaceutical Technology
APRIL 19, 2024
Pharmaceutical Compliance Management Software Is Designed to Enhance Patient Safety. Download the Free Buyer’s Guide Here.

Pharma Mirror
NOVEMBER 7, 2023
Navigating regulatory compliance in the pharmaceutical industry is crucial to ensure the safety, efficacy, and quality of pharmaceutical products. Good Manufacturing Practices The post Navigating Regulatory Compliance in the Pharmaceutical Industry appeared first on Pharma Mirror Magazine.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MARCH 26, 2024
KOLs interviewed by GlobalData emphasised urgent action to address inconsistent drug compliance rates in the osteoporosis disease landscape.

pharmaphorum
JANUARY 22, 2024
Pharma waste management is crucial for balancing cost, compliance, and environmental responsibility in the healthcare industry. Explore strategies and best practices for effectively managing pharmaceutical waste, while ensuring regulatory compliance and minimising environmental impact.

XTalks
JULY 9, 2024
In the dynamic landscape of clinical trials, ensuring compliance and maintaining high-quality standards are paramount. The field of quality and compliance in clinical trials is undergoing significant transformations driven by technological advancements, regulatory changes and evolving industry practices.

pharmaphorum
APRIL 30, 2024
Discover strategies for enhancing compliance and resilience in pharmaceutical supply chains through effective monitoring, due diligence, and understanding the impact on the industry. Find out more.

Pharmaceutical Technology
AUGUST 22, 2023
Qualifyze has secured $12m to improve supply chain compliance in the pharmaceutical sector using technological and audit data.

pharmaphorum
JULY 17, 2024
Discover how artificial intelligence is revolutionising drug development and compliance in the pharmaceutical industry with these four impactful ways.

Pharma Mirror
JANUARY 3, 2022
They can deal with other healthcare franchises, which also need compliance. There are more reasons why health professionals should have a HIPAA compliance program in their clinics and offices. The post 5 HIPAA Compliance Tips For Healthcare Professionals appeared first on Pharma Mirror Magazine.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 15, 2023
The National Accreditation Board for Testing and Calibration Laboratories (NABL) has issued clarification on NABL accredited Conformity Assessment Body (CAB) combined International Laboratory Accreditation Co-operation (ILAC) Mutual Recognition Arrangement (MRA) mark for effective compliance.

pharmaphorum
OCTOBER 12, 2022
Prior to selling drugs or medical products in any country, pharmaceutical companies must prove compliance and gain the regulatory approval required by the country in which the goods will be distributed in. So, with mandates in place, how do manufacturing facilities meet these compliance goals? The dominion of data.
Pharmaceutical Technology
JUNE 23, 2023
CDMOs face growing challenges to keep up with QA and compliance. The regulatory framework governing medicine development is in flux.

World of DTC Marketing
SEPTEMBER 12, 2022
There are many reasons for non-compliance and non-adherence: Cost and affordability, lack of understanding/comprehension of advice, language barriers, cognitive abilities, fear of asking for clarification, or other reasons. The post Is Apple that naive when it comes to compliance? Oh, to live in an ideal world.

XTalks
JULY 18, 2024
This episode of the Xtalks Food Podcast features an interview with with Matt Brown, CEO of Wherefour, a cloud-based ERP (enterprise resource planning) platform that helps food and beverage companies, large and small, solve production and compliance issues. Matt discusses his background and what led him to lead Wherefour.

pharmaphorum
MAY 10, 2024
The 2nd mRNA Quality Control & Compliance Summit 2024 returns this June 25-27 Boston, MA. With world-class speakers from the likes of CureVac, Intellia Therapeutics, Zipcode Bio, Tessera Therapeutics, and many more

Pharmaceutical Commerce
JULY 25, 2023
With FDA’s compliance deadline only three months away, the tech-supply chain partnership is expected to help manufacturers and wholesalers meet the Nov. 27 deadline.

ACRP blog
JUNE 30, 2023
Clinical trial billing compliance—ensuring that charges for therapies and other study-related elements are sent to the appropriate party—is a key concern for academic medical centers and other research organizations. She concluded that “a proactive approach to clinical trial billing compliance can help mitigate these risks.”

ACRP blog
JANUARY 8, 2024
Few industries have a need to understand the complexities of compliance more than clinical research. The importance of compliance in clinical research cannot be stressed enough. This year’s Compliance Institute features more than 100 educational sessions across 14 unique learning tracks. This is a sponsored message.

Imperical Blog
DECEMBER 13, 2023
But here come the holidays – will your study participant compliance waver during these busy times? Patient recruitment, retention, and compliance with study… The post 3 Tips to Avoid the Holiday Season from Impacting Participant Compliance appeared first on Imperial Clinical Research Services Blog.

ACRP blog
FEBRUARY 28, 2024
To get the latest information and insights on a broad spectrum of topics, Health Care Compliance Association® (HCCA®) invites you to join us for our 28th Annual Compliance Institute , April 14–17. The city of Nashville, Tenn., Those who attend the virtual conference option can network with an anticipated 1,000 virtual attendees.

FDA Law Blog
JULY 22, 2024
The American Conference Institute (“ACI”) is holding its 2nd West Coast Editionof its Legal, Regulatory, and Compliance Forum on Cosmetics & Personal Care Products from September 25-26 at the Le Meridien Delfina, Santa Monica, California. s John W.M.

pharmaphorum
AUGUST 1, 2023
How pharma can improve regulatory compliance with AI-based technology Mike.Hammerton Tue, 01/08/2023 - 08:00 Bookmark this

Pharma Marketing Network
OCTOBER 13, 2023
Understanding digital compliance, navigating ad regulations, leveraging digital tools, and complying with pharma regulations are all important steps in ensuring successful marketing campaigns. Understanding Digital Compliance In order to stay compliant, pharma marketers must understand the digital compliance landscape.

Fierce Pharma
AUGUST 10, 2023
After Pfizer allegedly fired a compliance manager for raising the flag on potential fraud activity in China, the former employee is hitting back. Frank Han, Pfizer's former director of global compliance analytics, has filed a civil complaint against the drugmaker.

Outsourcing Pharma
OCTOBER 16, 2023
AI start-up, Leucine, claims ânot fit for purposeâ compliance protocol processes are hindering the ability to deliver life-saving drugs faster.
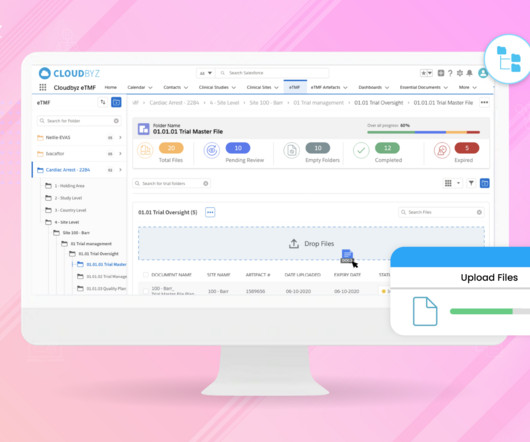
Cloudbyz
JUNE 5, 2023
In the fast-paced world of clinical research, managing essential Trial Master Files (TMFs) efficiently, while ensuring quality and compliance, is paramount. In this blog, we will explore how generative AI can revolutionize electronic Trial Master File (eTMF) management, unlocking new levels of efficiency, quality, and compliance.

Pharmaceutical Technology
JULY 15, 2024
AstraZeneca has obtained Health Canada’s Notice of Compliance (NOC) for Tagrisso (osimertinib) to treat NSCLC.

pharmaphorum
MAY 4, 2021
The life sciences history industry has some unique challenges when it comes to compliance. But if you think third party compliance risks are bad, don’t take your eye off fourth and fifth parties, says Allan Matheson. A decade ago, life sciences firms were early adopters of robust compliance technology — far ahead of other industries.

ACRP blog
JANUARY 11, 2023
Compliance can feel like a mild distraction at best, or a mass of red tape that slows forward progress. Health Care Compliance Association® (HCCA®) was established to do just that. As a nationally recognized association, HCCA is the authority on healthcare compliance and ethics, so you don’t have to be.

Pharmaceutical Technology
AUGUST 22, 2022
Fixed-dose combination (FDC) systems aim to increase patient compliance in therapies involving two or more active drugs. The post Compliance to convenience: understanding the latest innovations in capsule formulation appeared first on Pharmaceutical Technology. To find out more about Procaps softgels, download the whitepaper. [1]

FDA Law Blog
NOVEMBER 30, 2023
The 2023 Food and Drug Law Institute’s (“FDLI”) Enforcement, Litigation and Compliance Conference will boast two speakers from Hyman, Phelps & McNamara, P.C. (“HPM”), The conference will also include its annual panel discussion with FDA’s Center Compliance Directors. Blumberg Memorial Lecture. next week, on December 6-7, 2023.

WCG Clinical
MARCH 28, 2024
The post Ask the Experts: Your Coverage Analysis & Billing Compliance Questions Answered appeared first on WCG.

ProRelix Research
DECEMBER 28, 2023
Considering the crucial role that the information generated from clinical trials play in the approval of new drugs, biological, and medical devices, it is only logical that the data garnered […] The post Clinical Data Standardization in Clinical Trials: FDA Compliance in Clinical Data Management appeared first on ProRelix Research.

WCG Clinical
MARCH 7, 2024
Part Two Slides Download The post Beyond the Standard Coverage Analysis: Unpacking Challenging Billing Compliance Issues During Start-Up appeared first on WCG.
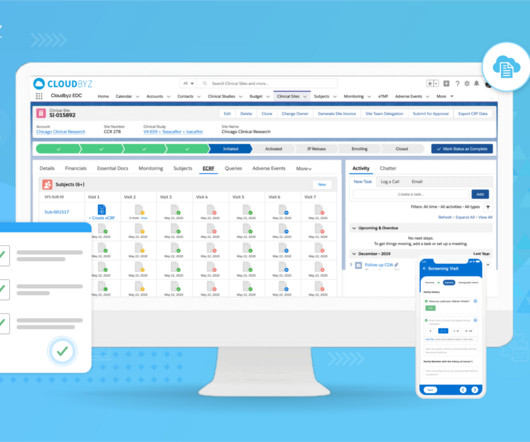
Cloudbyz
JUNE 16, 2023
Effective clinical trial data archiving is essential to ensure data integrity, regulatory compliance, and seamless access. Purpose of Clinical Trial Data Archiving: Compliance: Regulatory authorities require clinical trial data to be retained for a specific duration to demonstrate compliance with guidelines and regulations.

Cloudbyz
JUNE 10, 2023
21 CFR Part 11 is a crucial component of regulatory compliance in clinical trials and, more broadly, any industry subject to the regulations of the U.S. This blog post will serve as a comprehensive guide to understanding and implementing 21 CFR Part 11 compliance in your clinical trials. Food and Drug Administration (FDA).

Pharma Tutor
JULY 9, 2022
Data Integrity and Compliance With CGMP. Read more about Data Integrity and Compliance With CGMP Log in or register to post comments Read more about Data Integrity and Compliance With CGMP Log in or register to post comments Data integrity refers to the completeness, consistency, and accuracy of data. Sat, 07/09/2022 - 16:51.

BioSpace
FEBRUARY 26, 2023
Pay Transparency Backlash: the Harm of Reluctant Compliance 2/27/2023

WCG Clinical
FEBRUARY 23, 2024
Understanding-Billing-Compliance-Part-1-Slides Download The post Understanding Research Billing Compliance: Navigating the Basics and Beyond – Part One appeared first on WCG.

Advarra
OCTOBER 4, 2023
When it comes to clinical development, precision, compliance, and quality assurance are paramount. For clinical development organizations, an effective CAPA serves as an essential compass, directing a path towards continuous improvement while maintaining steadfast regulatory compliance.

Cloudbyz
APRIL 25, 2023
This comprehensive guide delves into the essentials of pharmacovigilance regulatory reporting, highlighting its significance in maintaining drug and device safety, and ensuring compliance with regulatory requirements. Different types of reports have specific timelines for submission, and delays may result in regulatory actions or penalties.

Imperical Blog
APRIL 11, 2023
This final installment explores clinical trial retention and compliance. Are you waiting too long to begin planning patient retention and… The post Hidden Causes of Clinical Trial Retention and Compliance Issues appeared first on Imperial Clinical Research Services Blog. Part one is here, and part two is here.

Imperical Blog
APRIL 11, 2023
This final installment explores clinical trial retention and compliance. Are you waiting too long to begin planning patient retention and… The post Hidden Causes of Clinical Trial Retention and Compliance Issues appeared first on Imperial Clinical Research Services Blog. Part one is here, and part two is here.

Outsourcing Pharma
AUGUST 14, 2023
LighthouseAI a company that specializes in compliance solutions for the pharmaceutical supply chain has announced a $2.25 million seed round, led by Healthy Ventures with participation from Bertelsmann Next.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content