The Benefits of Working for a Rapidly Growing Global Clinical Research Organization (CRO) like Medpace
XTalks
OCTOBER 18, 2022
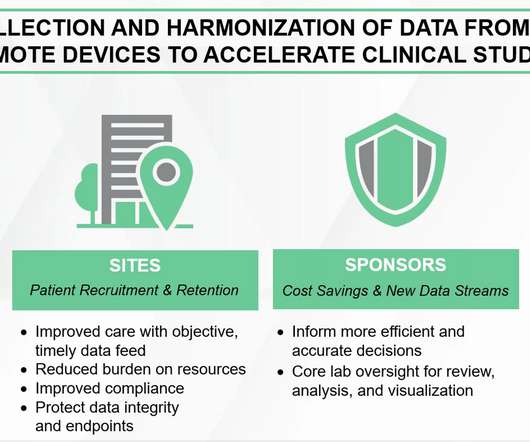
A clinical research organization (CRO) is a company that delivers outsourced services to plan, manage and execute clinical trials for biotechnology, pharmaceutical, and medical device companies. Read on to learn why life science and clinical research professionals are choosing a career with Medpace.














Let's personalize your content