The Challenges of Adopting Decentralized Clinical Trial Platforms
Cloudbyz
APRIL 17, 2024
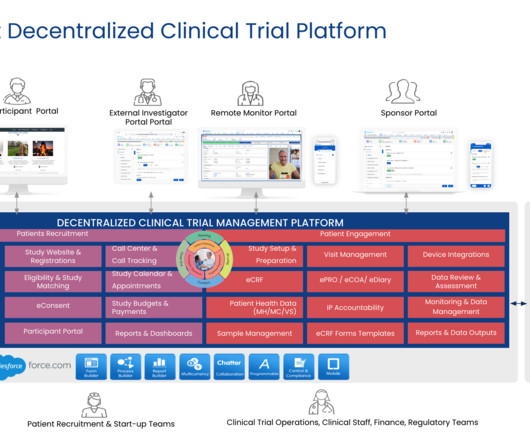
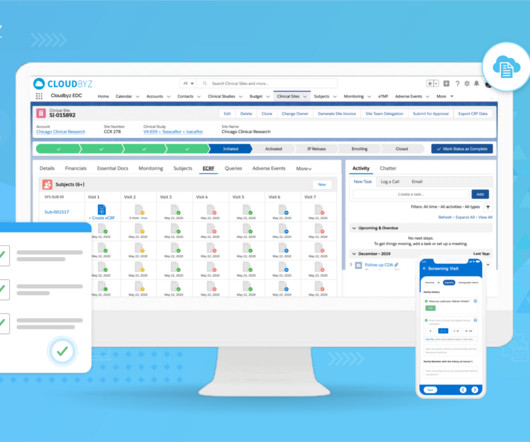
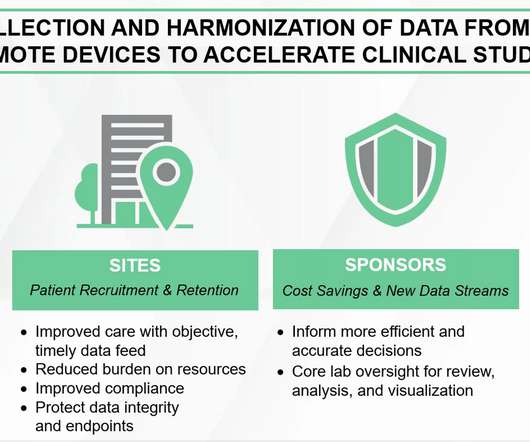
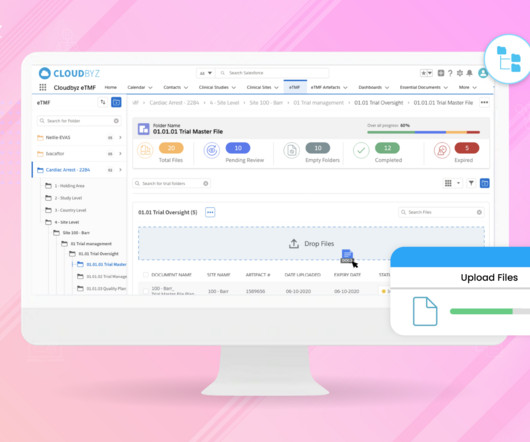
Decentralized clinical trial (DCT) platforms represent a paradigm shift in the way clinical research is conducted. By leveraging technology, DCTs aim to make clinical trials more accessible and convenient for patients, reduce costs and increase efficiency.
































Let's personalize your content