Top 40 Best-Selling Oncology Drugs in 2023 by 2022 Data
XTalks
FEBRUARY 5, 2024
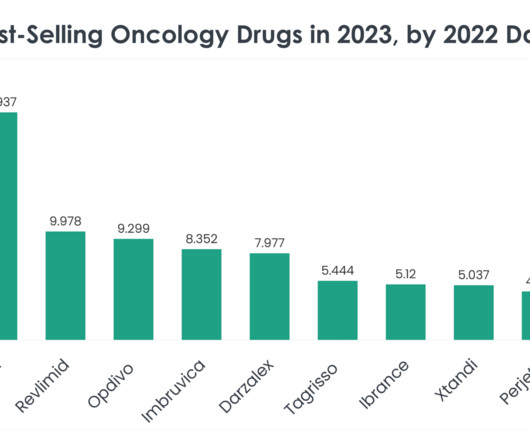
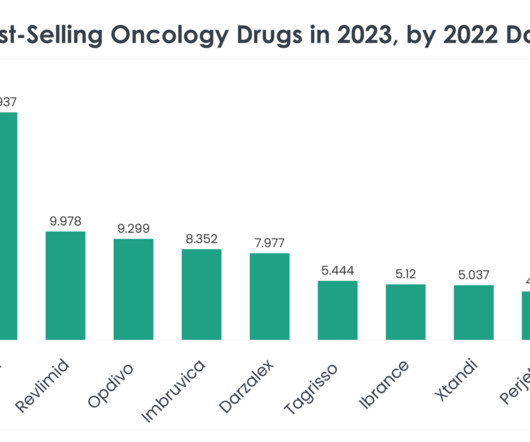
Read on to learn more about the top 40 best-selling oncology drugs in 2023, based on 2022 sales data. Nevertheless, Opdivo continues to hold strong and Bristol Myers Squibb filed an application with the FDA in December for Opdivo in combination with cisplatin chemotherapy as a first-line treatment in advanced urothelial carcinoma.











Let's personalize your content