Discovery reveals two potential drug targets against skin cancer
Drug Discovery World
APRIL 22, 2024
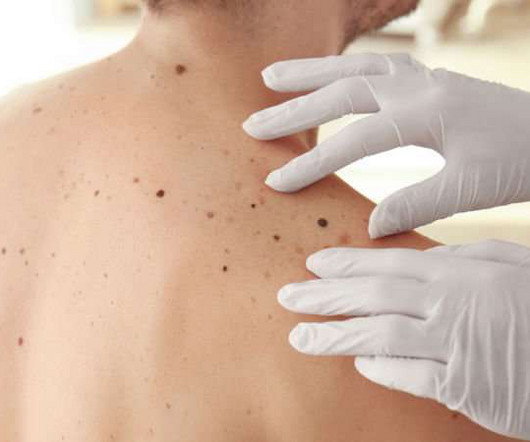
Scientists have discovered how skin cancer cells shapeshift based on their environment – enabling them to spread through the body and cause a metastatic cancer. The discovery of two genes responsible for sensing the environment and adapting cell shape could offer potential new drug targets to stop cancer from metastasising. Cancer cells can change shape to move around the body, becoming drill-shaped to ‘poke’ through dense tissue like bone, or round and squishy to squeeze through soft tissues an









































Let's personalize your content