Russia’s focus on domestic pharma production could shield it from sanctions’ effects
Pharmaceutical Technology
JULY 4, 2022
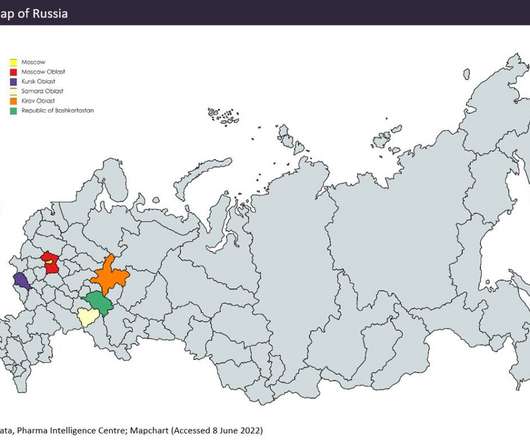
Compared with other emerging pharmaceutical markets (such as India and China, covered in previous Emerging Market Outsourcing Reports), Russian facilities lack many US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approvals, meaning Russian manufacturing is more focused on the domestic market.









Let's personalize your content