Radical Vaccine Strategy Could Help Quash Parasite Afflicting Millions
AuroBlog - Aurous Healthcare Clinical Trials blog
APRIL 11, 2024
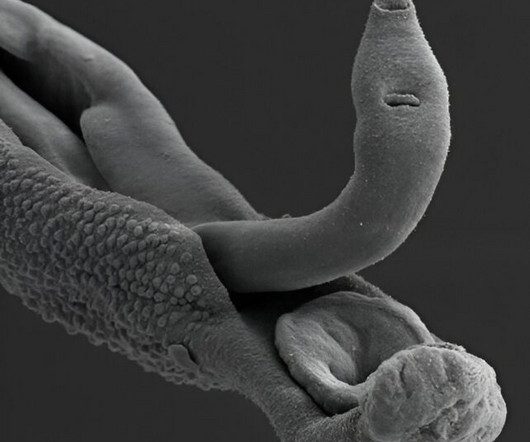
Using viruses that infect bacteria to detect proteins sprouted by a notorious parasite, scientists have honed in on possible vaccine targets for schistosomiasis, a neglected tropical disease that currently affects an estimated 600 million people worldwide, causing 280,000 deaths per year.























Let's personalize your content