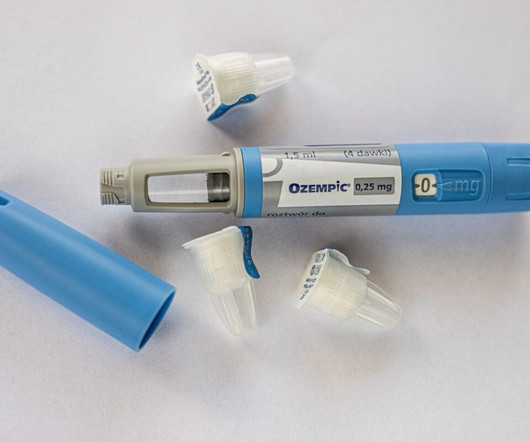
FDA Warns About Compounded Versions of Ozempic and Wegovy
XTalks
JUNE 5, 2023
Ozempic and Wegovy, which contain the active ingredient semaglutide, were approved by the US Food and Drug Administration (FDA). During a drug shortage, compounders might be allowed to prepare a compounded version of the drug provided they meet specific requirements of the Federal Food, Drug, and Cosmetic (FD&C) Act.










Let's personalize your content