The criticality of compliance
pharmaphorum
OCTOBER 12, 2022
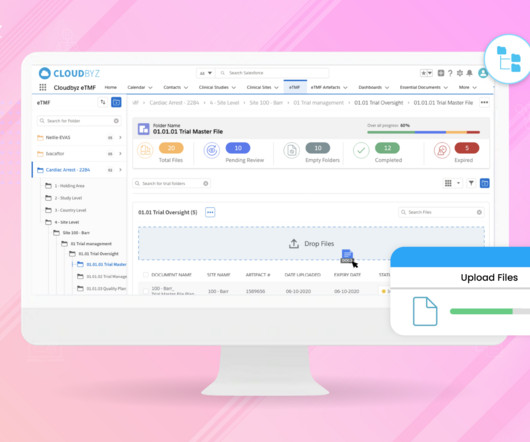
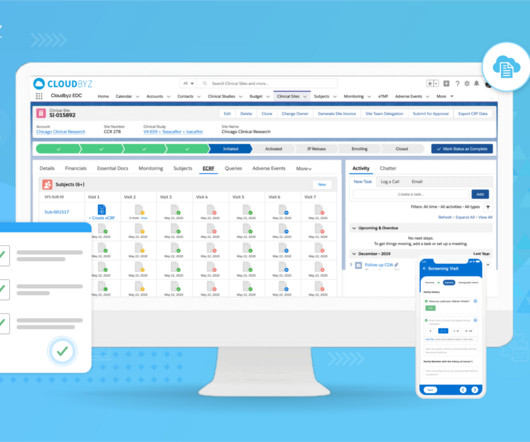
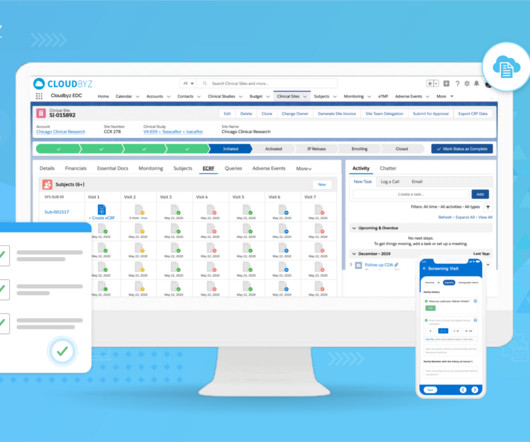
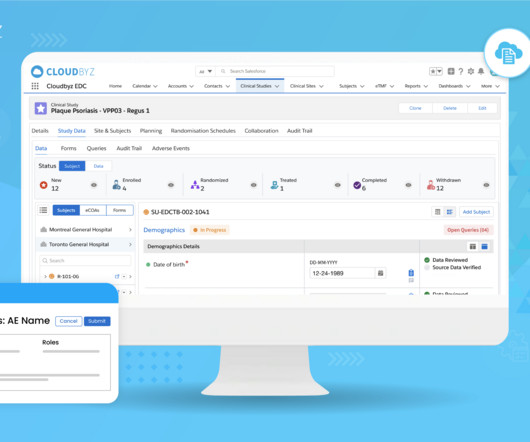
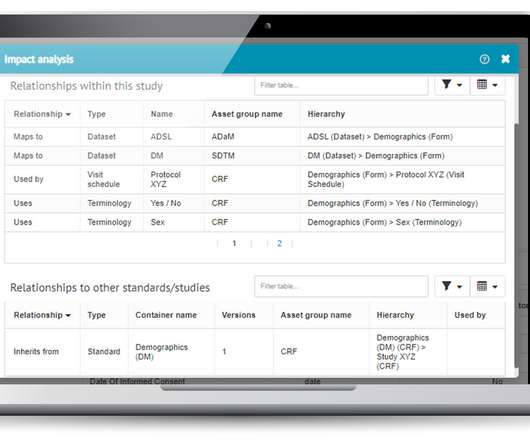
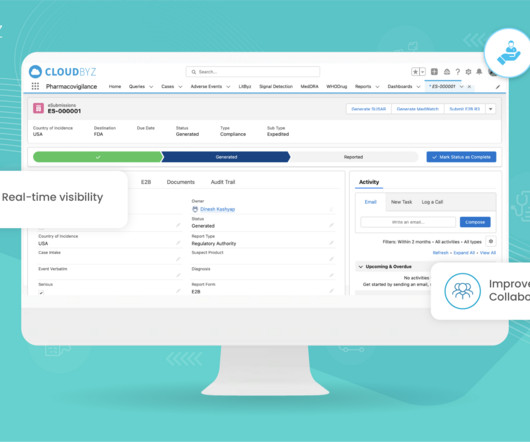
Prior to selling drugs or medical products in any country, pharmaceutical companies must prove compliance and gain the regulatory approval required by the country in which the goods will be distributed in. So, with mandates in place, how do manufacturing facilities meet these compliance goals? The dominion of data.











































Let's personalize your content