This week in drug discovery (8-12 April)
Drug Discovery World
APRIL 12, 2024
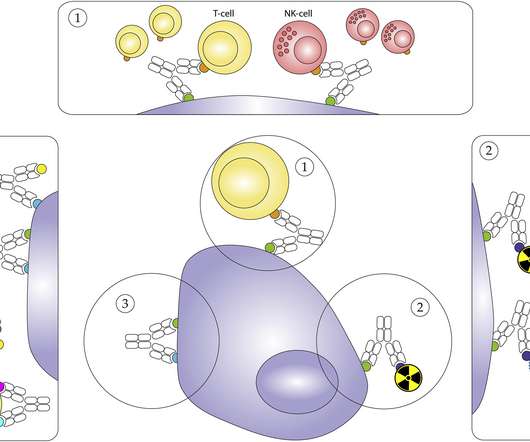
The top stories: Genetically engineered dendritic cells enhance lung cancer therapy A study by researchers at the UCLA Health Jonsson Comprehensive Cancer Center in the US suggests that injecting engineered dendritic cells directly into cancerous lung tumours can help promote a stronger immune response.
















Let's personalize your content