Untangling the Complexities of Cell and Gene Therapy Clinical Trials: A Supply Chain Perspective
Pharmaceutical Technology
MAY 24, 2023
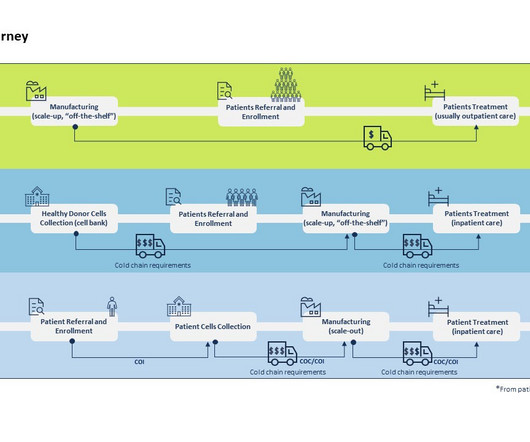
Every major pharma company is now involved in CGT development which has resulted in the approval of 28 therapies by the FDA thereby making CGT no longer a niche category of therapies. What are the general differences in the supply chain of CGT vs. traditional clinical trials? Differences in CGT vs traditional trials supply chain.









Let's personalize your content