Prefilled syringes and vaccines: Optimising parenteral packaging for the next pandemic
Pharmaceutical Technology
NOVEMBER 15, 2022
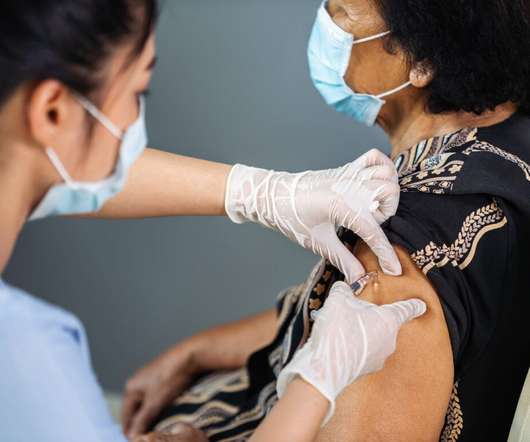
When it comes to vaccines and other high-value biologics, it is essential that effective formulations go hand in hand with safe delivery. An example is the ability of a patient suffering from venous thrombosis to perform their own injections of heparin – an anticoagulant often packaged in a prefilled syringe.












Let's personalize your content