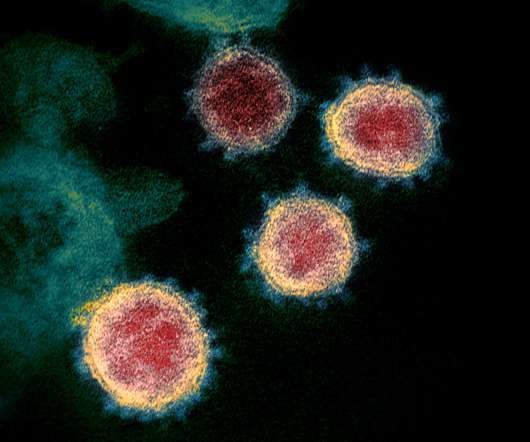
BioSig abandons COVID-19 drug trial on safety concerns
pharmaphorum
OCTOBER 27, 2020
The company opted to test merimepodib in the combination trial first as that had the greatest potential to make a difference to patients, but the safety issue means that it has decided to halt development of the drug altogether. The post BioSig abandons COVID-19 drug trial on safety concerns appeared first on.








Let's personalize your content