25 Reasons Why Pharmaceutical Companies Need a Unified Clinical Trial Management Platform
Cloudbyz
OCTOBER 19, 2023
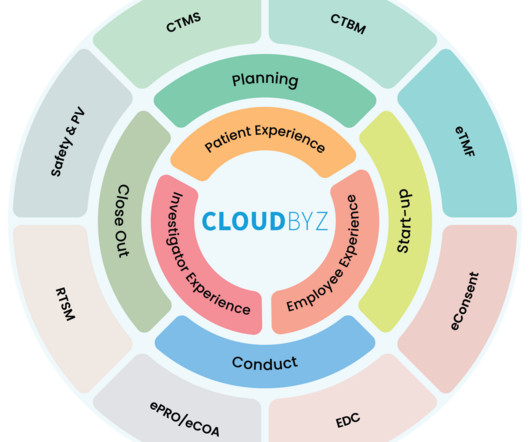
Clinical trials are the backbone of drug development, and managing these trials efficiently is paramount. In recent years, the need for a unified clinical trial management platform has become increasingly evident. Cost Efficiency: Centralized data management reduces administrative overhead, minimizing trial costs.

















Let's personalize your content