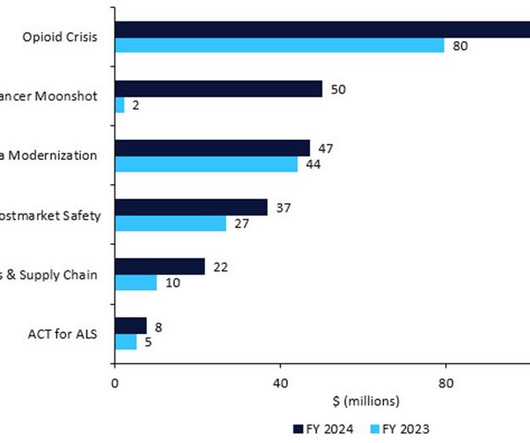
FDA $7bn plans for 2024: disclose contract manufacturers, restart Cancer Moonshot
Pharmaceutical Technology
MARCH 30, 2023
The US FDA has revealed its detailed budget proposal for FY2024, which would require pharma companies to name their active pharmaceutical ingredient (API) suppliers, restart President Biden’s Cancer Moonshot, inject cash into amyotrophic lateral sclerosis (ALS) research, and enforce stricter rules around manufacturing, recalls, and shortages.










Let's personalize your content