NICE, NHS England unveil new pathway for medtech
pharmaphorum
MAY 24, 2024
NICE and NHS England are seeking feedback on proposed new rules and commissioning principles for medtech products.

pharmaphorum
MAY 24, 2024
NICE and NHS England are seeking feedback on proposed new rules and commissioning principles for medtech products.

Bio Pharma Dive
MAY 24, 2024
But the head of the FDA’s CBER office didn’t tip where the agency stands on potentially broadening use of Sarepta’s Duchenne gene therapy Elevidys.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 24, 2024
The US FDA's EMDAC is set to hold a meeting to assess the benefit-risk profile of Novo Nordisk's insulin icodec, to treat type 1 diabetes.

Fierce Pharma
MAY 24, 2024
While it’s hard to overstate the success Novo Nordisk’s GLP-1 franchise has already achieved, the company’s latest deep dive into semaglutide data in chronic kidney disease (CKD) could help set a n | Early Friday, Novo shared full, positive results from the FLOW trial assessing the ability of once-weekly semaglutide 1.0 mg to help combat major kidney outcomes such as kidney failure, loss of kidney function and death from kidney or cardiovascular causes in people with Type 2 diabetes and chronic

Pharmaceutical Technology
MAY 24, 2024
Shares in Merus rose by 15% in premarket trading, following the announcement of positive Phase II data to be presented at ASCO.

pharmaphorum
MAY 24, 2024
Exciting advancements in the world of clinical trials - Arecor Therapeutics, Faron, AstraZeneca, Volta Medical, J&J, Pneumagen, and Nimbus Therapeutics make headlines. Dive into this week's top stories impacting the future of medicine.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

pharmaphorum
MAY 24, 2024
AffyImmune Therapeutics has recorded what it says is the first documented complete response in a patient with a solid tumour treated with CAR-T therapy. The case comes from a phase 1 study of its ICAM-1 targeting CAR-T autologous candidate AIC100 in patients with relapsed/refractory poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC), which will be reported at the ASCO congress next week.

Pharmaceutical Technology
MAY 24, 2024
The clinical-stage biotech will share preliminary safety and efficacy data with its ADC at the 2024 ASCO meeting.

Fierce Pharma
MAY 24, 2024
In a change of pace from the recent COVID-related business struggles for Pfizer, the drugmaker has picked up a valuable reward from the FDA in the form of a priority review voucher (PRV). | The FDA awarded the voucher based on Paxlovid's status as a countermeasure to a material medical threat, according to a notice in the Federal Register.

Pharmaceutical Technology
MAY 24, 2024
Grey Wolf Therapeutics has concluded an oversubscribed $50m in Series B financing expansion to advance its antigen modulation technology.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
MAY 24, 2024
This week’s round-up of biotech financings is led by a $120 million round for UK-based antibody-drug conjugate (ADC) developer Pheon, with SixPeaks also pulling in nine figures and smaller rounds for Progentos, LabGenius, and Grey Wolf, and Imvax.
Pharmaceutical Technology
MAY 24, 2024
Affecting more than 162,000 patients worldwide, global clinical trials are researching innovative new treatments for cystic fibrosis.

Pharma Times
MAY 24, 2024
The lifelong autoimmune disease affects more than 150,000 people living in the UK

Pharmaceutical Technology
MAY 24, 2024
MSD licenced the antibody-drug conjugate (ADC) therapy, sacituzumab tirumotecan, from China-based Sichuan Kelun-Biotech in 2022.

pharmaphorum
MAY 24, 2024
Shares in Merus spike on ASCO abstract on phase 2 trial of EGFRxLGR5 bispecific petosemtamab in head and neck cancer.

Pharmaceutical Technology
MAY 24, 2024
Biocon has entered an exclusive licence and supply agreement with Handok to commercialise synthetic Liraglutide in South Korea.

Pharma Times
MAY 24, 2024
The conference will take place on 23 to 24 October at Vienna AirportCity
Pharmaceutical Technology
MAY 24, 2024
Shares in the pharma company rose 40% in premarket trading.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
MAY 24, 2024
AstraZeneca's CEO wants to tap into China's innovation explosion. The company inked an oncology antibody deal with Harbour BioMed, and it plans to build a $1.5 billion ADC plant in Singapore. | AstraZeneca's CEO wants to tap into China's innovation explosion. The company inked an oncology antibody deal with Harbour BioMed, and it plans to build a $1.5 billion ADC plant in Singapore.

Cloudbyz
MAY 24, 2024
Selecting the right Electronic Data Capture (EDC) system is vital for the success of clinical trials. An effective EDC system streamlines operations, ensures data quality, and enhances regulatory compliance while prioritizing patient engagement. To help you make an informed decision, we’ve created a comprehensive buyer’s checklist. This checklist covers essential features such as product/ user administration, data management, regulatory compliance, and user support.

Pharma Times
MAY 24, 2024
JHU083 reduced tumour growth and triggered tumour cell death in mice

Drug Patent Watch
MAY 24, 2024
Annual Drug Patent Expirations for CHILDREN%27S+ADVIL+ALLERGY+SINUS For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit www.DrugPatentWatch.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Drug Discovery World
MAY 24, 2024
News round-up for 20-24 May by DDW Senior Digital Content Editor Diana Spencer. The headline news this week emphasises the importance of shared expertise and collaboration, whether that be between nations, companies or research institutions, to enable the greatest advances in drug discovery. The top stories: Partners explore precision medicine approach to heart disease PlaqueTec and the Welch research group at the Babraham Institute have announced the completion of their collaboration to evaluat

Drug Patent Watch
MAY 24, 2024
Annual Drug Patent Expirations for CHILDREN%27S+ADVIL-FLAVORED For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit www.DrugPatentWatch.

XTalks
MAY 24, 2024
World Hunger Day 2024 is fast approaching, offering an opportunity to address global food insecurity. This day, observed annually on May 28, brings together organizations, communities and individuals to fight hunger worldwide. World Hunger Day was founded by The Hunger Project in 2011. This global initiative aims to raise awareness about the hundreds of millions of people who suffer from chronic hunger.

Drug Patent Watch
MAY 24, 2024
Annual Drug Patent Expirations for MIRVASO Mirvaso is a drug marketed by Galderma Labs Lp and is included in one NDA. It is available from one supplier.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
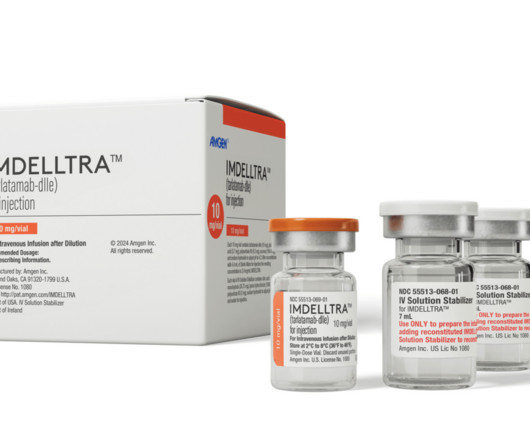
XTalks
MAY 24, 2024
The US Food and Drug Administration (FDA) has approved Amgen’s Imdelltra (tarlatamab-dlle) for the treatment of extensive-stage small cell lung cancer (ES-SCLC) in patients who have experienced disease progression after platinum-based chemotherapy. The approval is based on the overall response rate and duration of response observed in the DeLLphi-301 study , with continued approval depending on the demonstration of clinical benefit in a future confirmatory trial(s).

Drug Discovery World
MAY 24, 2024
etherna has entered a research collaboration with Hasselt University (UHasselt) to develop an mRNA based treatment for autoimmune disorders. Funded by Flanders Innovation & Entrepreneurship (VLAIO), the project aims to combine etherna’s vast expertise in developing customised mRNA and lipid-based nanoparticle technologies for immuno-modulation with UHasselt’s research into autoimmune diseases with a particular focus on multiple sclerosis.

Drug Patent Watch
MAY 24, 2024
Annual Drug Patent Expirations for LATISSE Latisse is a drug marketed by Abbvie and is included in one NDA. It is available from one supplier.

pharmaphorum
MAY 24, 2024
The biosimilars dance has big pharma and regulators tangled in a complex patent battle. At stake: billions in profits and affordable access to vital meds.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content