The New Frontier in Drug Development: Understanding Biologics and Small Molecules
Cloudbyz
MARCH 5, 2024
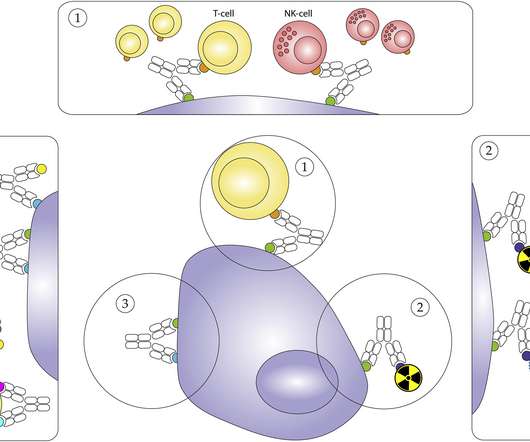
In the intricate dance of drug discovery and development, two protagonists emerge as the cornerstones of modern medicine: biologics and small molecules. They are often used to mimic or enhance natural biological processes, such as immune responses. Their small size allows them to distribute widely throughout the body.



















Let's personalize your content