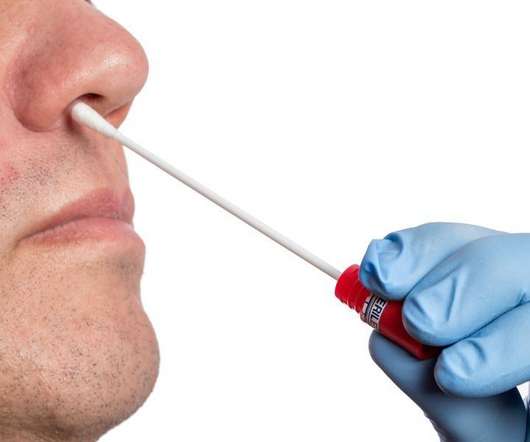
FDA Approves First Rapid COVID Test for Home Use
The Pharma Data
NOVEMBER 18, 2020
. “Today’s authorization for a complete at-home test is a significant step toward FDA’s nationwide response to COVID-19,” Jeff Shuren, director of FDA’s Center for Devices and Radiological Health, said in a statement. But the new at-home test relies on similar principles.










Let's personalize your content