Team studies immune response, proteins in blood of young adults who develop rare complication after COVID vaccination
Medical Xpress
JANUARY 4, 2023
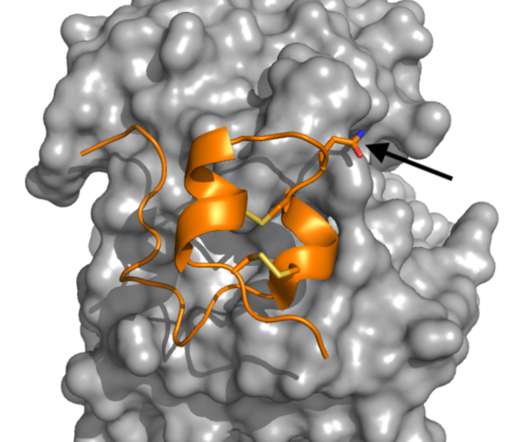
Myocarditis, a condition in which the heart muscle becomes inflamed, is a rare complication that can occur after mRNA COVID vaccination. It's estimated that roughly 18 cases occur in every 1 million vaccine doses administered, making it so rare that it is challenging to find cases to investigate.



























Let's personalize your content