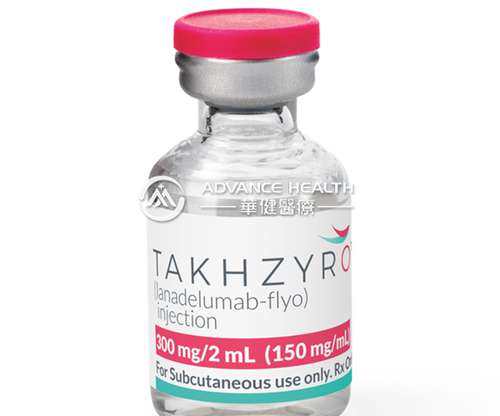
TAKHZYRO® (lanadelumab) for Hereditary Angioedema Patients to be Presented at European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress
The Pharma Data
JULY 11, 2021
TAKHZYRO is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology. Depending on regional marketing authorization, TAKHZYRO is available as a 300 mg dose in a vial or pre-filled syringe. TAKHZYRO is a fully human monoclonal antibody that specifically binds and decreases plasma kallikrein activity.








Let's personalize your content