J&J builds case for antidepressant Spravato with head-to-head trial
pharmaphorum
NOVEMBER 24, 2022
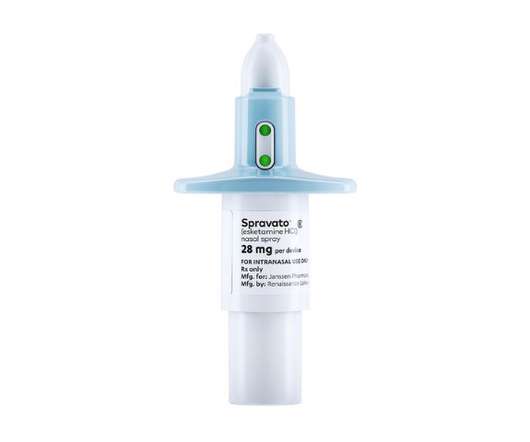
Johnson & Johnson’s Spravato therapy for treatment-resistant depression (TRD) hasn’t made the impact the company hoped for in the market, and it is looking to new clinical results to accelerate its take-up. The post J&J builds case for antidepressant Spravato with head-to-head trial appeared first on.










Let's personalize your content