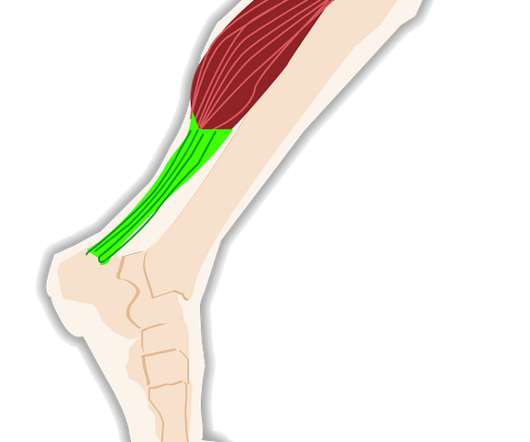
FDA Approves Expanded BOTOX® (onabotulinumtoxinA) Label to Include Eight New Muscles to Treat Adults with Upper Limb Spasticity
The Pharma Data
JULY 29, 2021
BOTOX ® was first approved by the FDA in 1989 for two rare eye muscle disorders – blepharospasm and strabismus in adults. Patients treated for overactive bladder: In clinical trials, 36 of the 552 patients had to self-catheterize for urinary retention following treatment with BOTOX ® compared to 2 of the 542 treated with placebo.












Let's personalize your content