Ligelizumab by Novartis for Food Allergy: Likelihood of Approval
Pharmaceutical Technology
FEBRUARY 7, 2024
Ligelizumab is under clinical development by Novartis and currently in Phase III for Food Allergy.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
FEBRUARY 7, 2024
Ligelizumab is under clinical development by Novartis and currently in Phase III for Food Allergy.

Pharmaceutical Technology
FEBRUARY 19, 2024
Allergen for Peanut Allergy is under clinical development by ALK-Abello and currently in Phase II for Peanut Allergy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

VirTrial
FEBRUARY 28, 2024
If you missed Signant’s February 2024 webinar about optimizing patient-reported outcomes measurement strategy throughout oncology drug development, we’ve summarized the key takeaways and provided a link for you to watch the recording. Watch the recording You can watch the recorded webinar here.

Pharmaceutical Technology
JUNE 10, 2023
TAS-117 is under clinical development by Taiho Pharmaceutical and currently in Phase II for Chondrosarcoma. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Pharmaceutical Technology
JUNE 10, 2023
TAS-117 is under clinical development by Taiho Pharmaceutical and currently in Phase II for Chondrosarcoma. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.

Clinical Trial Podcast
SEPTEMBER 26, 2022
To learn more about the complexities in early phase clinical trials, I invited Dr. Oren Cohen , President of Clinical Pharmacology Services and Chief Medical Officer at Labcorp Drug Development on the show. Dr. Cohen has more than 30 years of healthcare experience which includes his work on clinical development.
pharmaphorum
SEPTEMBER 1, 2020
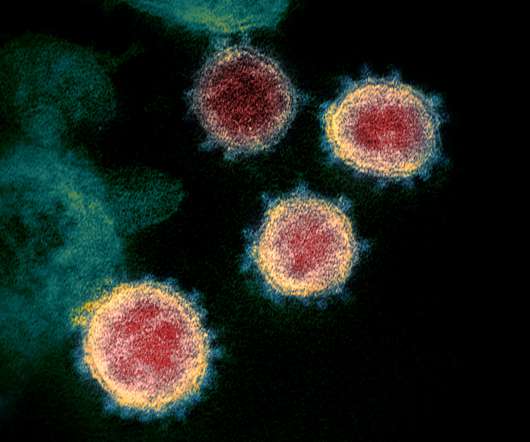
AstraZeneca has expanded development of COVID-19 vaccine AZD1222 into the US, beginning a phase 3 clinical trial across all adult age groups. Results from the late-stage trials are anticipated later this year, depending on the rate of infection within clinical trial communities.
XTalks
SEPTEMBER 21, 2023
This groundbreaking endeavor has received support from the National Institute of Allergy and Infectious Diseases (NIAID). Since 2004, the NIAID has been championing the discovery and development of the cytomegalovirus (CMV) vector used to deliver the HIV vaccine material to the immune system without causing disease. “HIV

pharmaphorum
DECEMBER 20, 2021
Biosimilars haven’t been approved yet but are in clinical development at the likes of Glenmark, BiosanaPharma and Celltrion. He said however that the company will “continue to evaluate the potential for ligelizumab to bring benefit to patients in the areas of chronic inducible urticaria and food allergy.”
The Pharma Data
APRIL 14, 2021
Roche’s Chief Medical Officer and Head of Global Product Development. Expanding treatment options for personalised care and self-management is always welcome news for the patient community,” said Kenneth Mendez, CEO and President, Asthma and Allergy Foundation of America. Approximately 460,000 patients have been treated in the U.S.
The Pharma Data
AUGUST 24, 2020
The treatment is being researched by scientists collaborating from American Gene Technologies, the Laboratory of Immunoregulation and the National Institute of Allergy and Infectious Diseases.

The Pharma Data
JANUARY 12, 2021
Dr Graham is a medicines development expert and Infectious Diseases Epidemiologist with global Biotech and Pharma R&D experience in Phase I-IV therapeutics as well as in-vivo & in-vitro diagnostics, across many modalities. He has in depth Global Development Expertise (e.g. NEW YORK and LONDON, Jan. Dr. Graham earned an M.D.,

Pharma Marketing Network
APRIL 27, 2021
Rod MacKenzie, PhD, is Chief Development Officer and Executive Vice President for Pfizer. Rod joined Pfizer in Sandwich, UK as a Research Scientist and conducted medicinal chemistry research in the cardiovascular, GI, Sexual Health, Urology and Allergy & Respiratory diseases. HBA Honorable Mentor 2020-21, Dr. Rod MacKenzie.

World of DTC Marketing
NOVEMBER 19, 2021
government has provided Moderna with nearly $10 billion in taxpayer money for research and development and the purchase of 500 million doses of this mRNA COVID-19 vaccine. This includes almost the entire cost of clinical development. billion for the development of its vaccine and to pay for doses once approved.

XTalks
NOVEMBER 6, 2020
Canadian clinical-stage biotech company Symvivo Corporation has developed an oral COVID-19 vaccine that entered clinical trials this week. The first healthy volunteer was dosed with the vaccine in Australia as part of the bacTRL-Spike COVID-19 Phase I clinical trial. Related: Red Meat Allergy Test Gets FDA Clearance. “We

The Pharma Data
AUGUST 7, 2020
As the pharma industry stands firm in its commitment to advance the sector to fight Covid-19, news has emerged from the European Commission who intend to streamline the development of therapies using genetically modified organisms to treat Covid-19. Dr. Francis S.

The Pharma Data
APRIL 4, 2022
The potential use of Dupixent in EoE is currently under clinical development, and the safety and efficacy have not been fully evaluated by any regulatory authority. Dupilumab Development Program. Dupilumab is being jointly developed by Sanofi and Regeneron under a global collaboration agreement.

XTalks
OCTOBER 26, 2023
She has 23 years of experience working with clients to move vaccine candidates through the clinical development pipeline, including regulatory submission. There are other types of COVID vaccines as well, including an adenoviral vector (AAV)-based one from AstraZeneca and a protein-based vaccine developed by Novavax.

The Pharma Data
SEPTEMBER 9, 2021
PT027 is a potential first-in-class inhaled, fixed-dose combination of albuterol, a short-acting beta2-agonist (SABA), and budesonide, an inhaled corticosteroid (ICS), being developed by AstraZeneca and Avillion. 2 Inflammation is a distinctive feature of asthma 3 and plays a key role in asthma symptoms, 4 exacerbations 5 and deaths.

Pfizer
NOVEMBER 2, 2022
The vaccine candidate combines Pfizer’s quadrivalent modRNA-based influenza vaccine candidate, qIRV (22/23), which is currently in Phase 3 clinical development , and Pfizer and BioNTech’s authorized Omicron-adapted bivalent COVID-19 BNT162b2 (Original/Omicron BA.4/BA.5) The companies will share the development costs.

The Pharma Data
OCTOBER 29, 2020
Cromos Pharma provides tailored and effective clinical trial solutions to support the development of drugs that transform healthcare. Clinical Development Strategy – we provide expert guidance on study design, favorable venues, local and global landscape to improve study outcomes. Source link.

The Pharma Data
AUGUST 5, 2021
Presenting the company’s half-year financial report, he highlighted how “we have achieved major successes in developing and launching drugs, some of which have blockbuster potential. The platform already has potentially ground-breaking medical innovations in clinical development, such as a therapy for the treatment of Parkinson’s.

Pfizer
SEPTEMBER 28, 2022
COMIRNATY, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products, including innovative medicines and vaccines. 5) could cause a severe allergic reaction.

Pfizer
JULY 26, 2022
The Pfizer-BioNTech COVID-19 Vaccine, BNT162b2, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer. Tell your vaccination provider about all of the vaccine recipient’s medical conditions, including if the vaccine recipient: has any allergies. IMPORTANT SAFETY INFORMATION . has a fever.

Pfizer
DECEMBER 8, 2022
Fast Track is a process designed to facilitate the development and expedite the review of new drugs and vaccines intended to treat or prevent serious conditions and address unmet medical need. Tell your vaccination provider about all of your medical conditions, including if you: have any allergies. have a fever.

Pfizer
JULY 19, 2022
The Pfizer-BioNTech COVID-19 Vaccine, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer. Tell your vaccination provider about all of the vaccine recipient’s medical conditions, including if the vaccine recipient: has any allergies. IMPORTANT SAFETY INFORMATION. has a fever.

Pfizer
JULY 8, 2022
COMIRNATY, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer. Tell your vaccination provider about all of your medical conditions, including if you: have any allergies. IMPORTANT SAFETY INFORMATION . have a fever. have a bleeding disorder or are on a blood thinner.

Pfizer
SEPTEMBER 16, 2022
COMIRNATY, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer. Tell your vaccination provider about all of your medical conditions, including if you: have any allergies. Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), have a fever.

Pfizer
JULY 8, 2022
COMIRNATY, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer. IMPORTANT SAFETY INFORMATION Tell your vaccination provider about all the vaccine recipient’s medical conditions, including if the vaccine recipient: has any allergies. has a fever. has received another COVID-19 vaccine.

XTalks
AUGUST 2, 2023
is a global pharmaceutical company, working across both developed and emerging markets. They are committed to capitalizing on growth opportunities primarily through the advancement of their own product pipeline and constantly improving their existing products, as well as through business development activities. Pfizer Inc.

XTalks
DECEMBER 24, 2020
Focusing on biologics, vaccine development and policy platforms in anticipation of an eventual outbreak is why we’re where we are today in terms of the positive side [of the pandemic].”. Most antibody drugs and vaccines have been developed to target parts of the spike protein. Pandemic Efforts. Diagnostics.
Drug Discovery World
JULY 20, 2022
Altasciences had originally developed the formulation and manufacturing processes to meet all of Alladapt’s Phase I and II clinical trial needs. Altasciences is building dedicated facilities for Alladapt to help with the company’s Phase III and commercial requirements. . Official comments . “We

Pfizer
OCTOBER 19, 2022
4-5) are based on BioNTech’s proprietary mRNA technology and were developed by both BioNTech and Pfizer. Tell your vaccination provider about all of your medical conditions, including if you: have any allergies. COMIRNATY® and its adapted vaccine variations (COMIRNATY® Original/Omicron BA.1 1 and COMIRNATY® Original/Omicron BA.4-5)
Drug Discovery World
MAY 1, 2024
A single injection of an experimental monoclonal antibody called L9LS was 77% effective at preventing malaria infection in children in Mali, according to the results of a mid-stage clinical trial. It was developed by scientists at the National Institutes of Health (NIH). “A

XTalks
SEPTEMBER 14, 2020
These companies include Moderna, a US biotech company which has formed a partnership with the National Institute of Allergy and Infectious Diseases (NIAID), Oxford University who has partnered with AstraZeneca, and Johnson & Johnson, Merck and Pfizer. helped protect COVID-19 patients against developing severe disease.

The Pharma Data
NOVEMBER 30, 2020
and is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. 75A50120C00034.

The Pharma Data
DECEMBER 23, 2020
24, 2020 /PRNewswire/ — COVAXX’s UB-612 is the first multitope, synthetic peptide-based COVID-19 vaccine candidate in clinical trials and it utilizes normal refrigeration (no freezing required) for distribution. COVAXX is currently conducting a Phase 1 clinical trial for the vaccine candidate. .
pharmaphorum
AUGUST 12, 2020
“It is absurd that Trump and [Health and Human Services Secretary Alex Azar] are touting $30 per course as a good deal for the American people, in light of the consistent and ongoing support from the US government towards mRNA-1273 research, development and manufacturing.”.
pharmaphorum
AUGUST 12, 2020
“It is absurd that Trump and [Health and Human Services Secretary Alex Azar] are touting $30 per course as a good deal for the American people, in light of the consistent and ongoing support from the US government towards mRNA-1273 research, development and manufacturing.”.

The Pharma Data
DECEMBER 23, 2020
company developing UB-612 a multitope peptide-based vaccine to fight COVID-19, today announced an exclusive agreement with Aurobindo Pharma to expand its global development and commercialization of UB-612 to India and the United Nations Children’s Fund (UNICEF) agency. Commenting on the development, Mr. N. HAUPPAUGE, N.Y.–(
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content