Vitamin E from palm oil useful in boosting immune response based on studies on liver cells
Scienmag
NOVEMBER 3, 2020
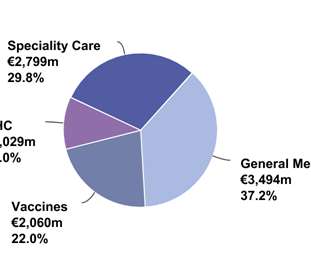
Ahmed Atia and colleagues is published in Current Pharmaceutical Biotechnology, 2020 Palm oil contains abundant quantities of vitamin E compounds, which include tocopherols and tocotrienols. While tocopherol is a widely known and researched compound, […].

























Let's personalize your content