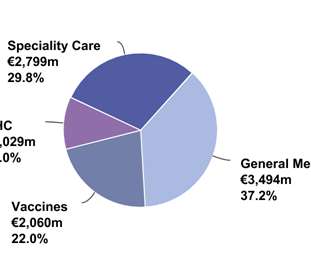
Switzerland Exercises Increased Option for 7.5 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273)
The Pharma Data
DECEMBER 8, 2020
Nasdaq: MRNA) a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients, today announced the Swiss Federal Government has increased its confirmed order commitment from 4.5 million doses of Moderna’s vaccine candidate against COVID-19 , mRNA-1273. “As



























Let's personalize your content