Infant antibiotic exposure can affect future immune responses toward allergies
Scienmag
APRIL 1, 2021
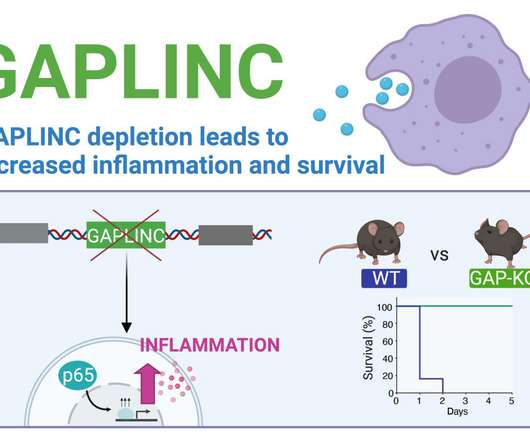
Early life exposure to antibotics in utero and through mother’s milk disrupts beneficial gut bacteria, compromising T-cell development, Rutgers research shows Exposure to antibiotics in utero and infancy can lead to an irreversible loss of regulatory T-cells in the colon-a valuable component of the immune system’s response toward allergens (..)



































Let's personalize your content