SK Bioscience gains UK authorisation for SKYCovion Covid-19 vaccine
Pharmaceutical Technology
MAY 30, 2023
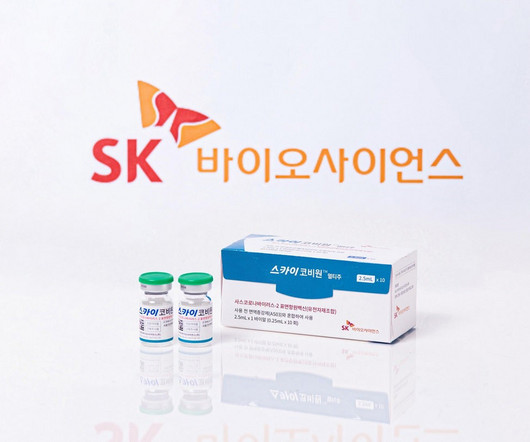
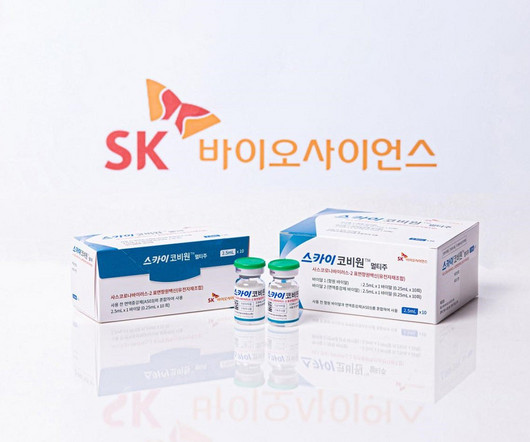
SK Bioscience has received marketing authorisation from the UK’s medicines and healthcare products regulatory agency (MHRA) for its Covid-19 vaccine, SKYCovion. The authorisation allows the distribution of the vaccine in Scotland, Wales and England.






























Let's personalize your content