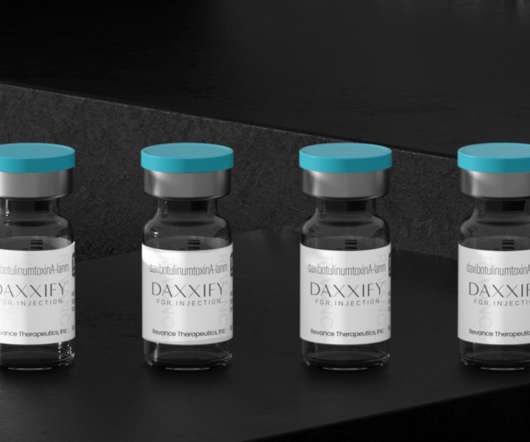
Daxxify, a New Anti-Wrinkle Drug and Botox Competitor is FDA-Approved
XTalks
SEPTEMBER 14, 2022
Daxxify was FDA-approved for similar cosmetic purposes as Botox and other neuromodulators like Dysport and Xeomin. One of the first options made available for such purposes was Botox from Allergan, brought to health practitioners’ offices in 1989. A Novel Botox Competitor. Bringing Daxxify into Offices.

















Let's personalize your content