Merit Medical to Participate at Four Conferences in September
BioTech 365
SEPTEMBER 2, 2020
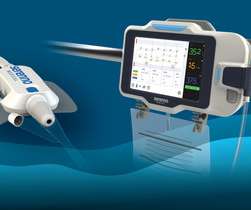
NASDAQ: MMSI), a leading manufacturer and marketer of proprietary disposable devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology, oncology, critical care and endoscopy, … Continue reading → SOUTH JORDAN, Utah, Sept.













Let's personalize your content