Mastering 21 CFR Part 11 Compliance: A Comprehensive Guide for Clinical Trials in the Digital Age
Cloudbyz
JUNE 10, 2023
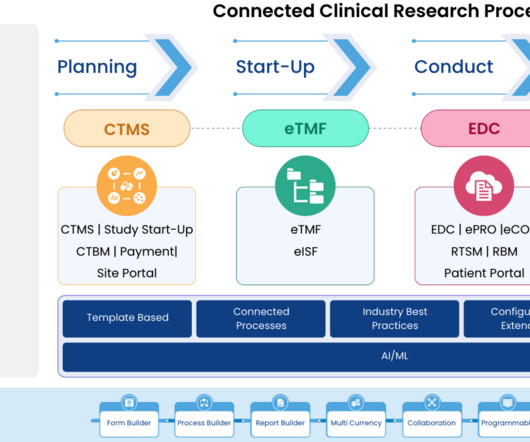
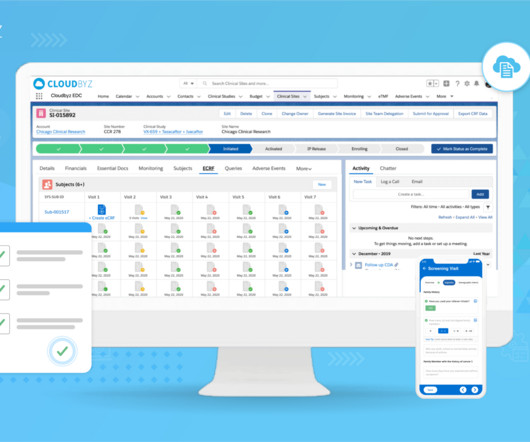
21 CFR Part 11 is a crucial component of regulatory compliance in clinical trials and, more broadly, any industry subject to the regulations of the U.S. This blog post will serve as a comprehensive guide to understanding and implementing 21 CFR Part 11 compliance in your clinical trials. Let’s explore.










Let's personalize your content