UK MHRA grants authorisation for Moderna’s Covid-19 booster vaccine
Pharmaceutical Technology
AUGUST 15, 2022
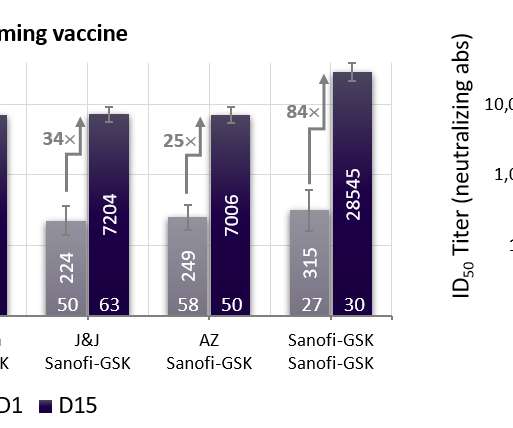
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted conditional authorisation for Moderna ’s Covid-19 booster vaccine, mRNA-1273.214 (Spikevax Bivalent Original/Omicron), for use in adults aged 18 years and above. 1) versus the 50µg mRNA-1273 booster dose. 1 variant as well as the original 2020 strain.”.









































Let's personalize your content