The next phase of decentralisation in clinical trials
Pharmaceutical Technology
APRIL 20, 2023
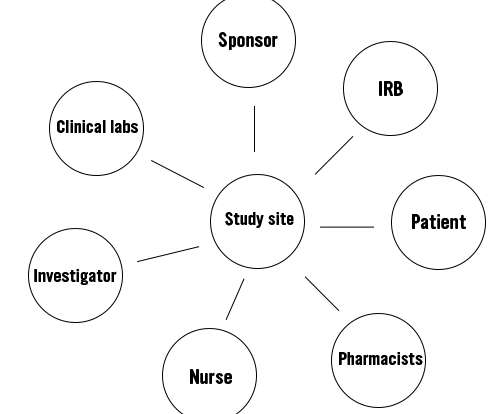
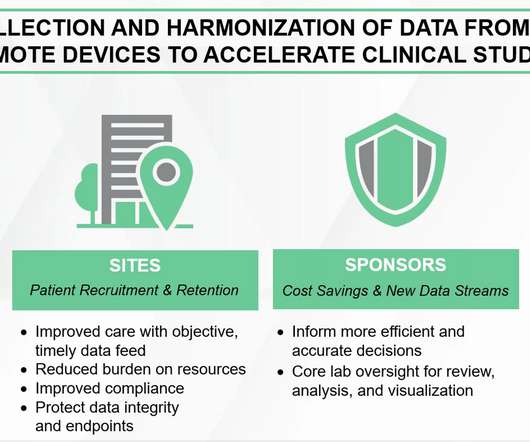
The face of clinical research is evolving. Rather than bringing patients to sites, study teams are now exploring ways they can bring the trial to patients using a varying combination of direct-to-patient drug shipments, home nursing, video conferencing, and electronic data collection. Drug development had to change.
















































Let's personalize your content