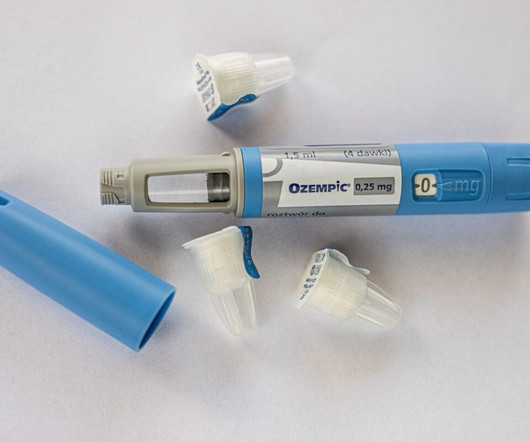
FDA Warns About Compounded Versions of Ozempic and Wegovy
XTalks
JUNE 5, 2023
Ozempic and Wegovy, which contain the active ingredient semaglutide, were approved by the US Food and Drug Administration (FDA). Semaglutide acts as a glucagon-like peptide-1 (GLP-1) receptor agonist, mimicking the GLP-1 hormone released in the gastrointestinal tract after eating.















Let's personalize your content