Moderna’s founder launches Laronde, promising new ‘Endless RNA’ drug class
pharmaphorum
MAY 11, 2021
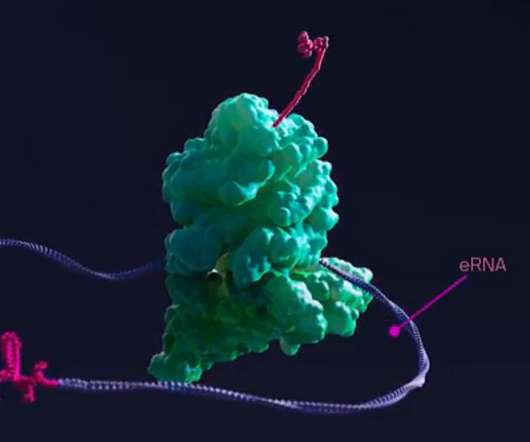
Flagship Pioneering, the VC fund run by Moderna’s co-founder Noubar Afeyan has launched a new biotech Laronde , with an ambitious plan to create a new class of drugs based on Endless RNA. The technology is designed to replace antibodies, which have become standard therapy in many diseases but are complicated and expensive to manufacture.




















Let's personalize your content