Untangling the Complexities of Cell and Gene Therapy Clinical Trials: A Supply Chain Perspective
Pharmaceutical Technology
MAY 24, 2023
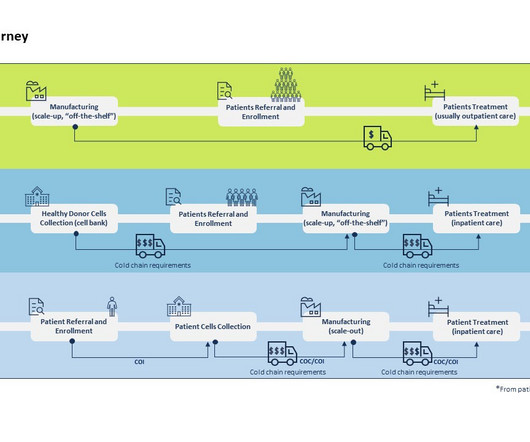
The ability to modify or introduce genetic material in human cells in such a precise and patient-centered manner clearly constitutes a breakthrough in personalized medicine. What are the general differences in the supply chain of CGT vs. traditional clinical trials? Differences in CGT vs traditional trials supply chain.










Let's personalize your content