CMO Moves: Regulatory catalysts for drug manufacturing-April
Pharmaceutical Technology
JULY 14, 2022
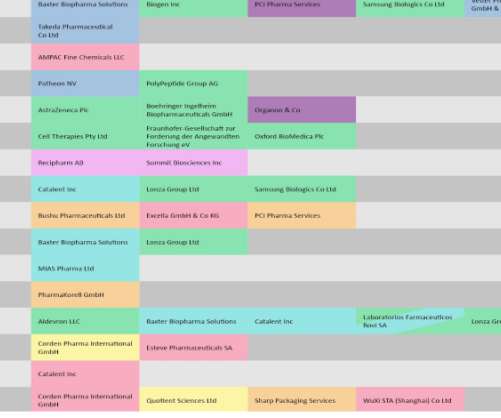
Pharmaceutical Technology looks at drugs and biologics with recent regulatory verdicts that will likely impact manufacturing volumes. In this ongoing series , we put a spotlight on contracts between pharma companies and contract manufacturing organizations (CMOs). Covid-19 vaccines stay in the spotlight.










Let's personalize your content